Abstract
Background: The prognosis of MM is determined by affected organs, tumor burden as measured by e.g., the international staging system (ISS), disease biology such as cytogenetic abnormalities, and response to therapy. The outcome of high-risk MM patients classified by ISS or adverse risk cytogenetics is not uniform and patients show heterogeneous survival. Recent insights into the pathogenesis of MM highlighted genome/transcriptome editing as well as inflammation as drivers for the onset and progression of MM. We hypothesized that inclusion of molecular features into risk stratification could potentially resolve the challenge of accurately distinguishing between high-risk and low-risk MM patients at initial diagnosis and improve outcome.
Aim: We aimed to create a simple molecular risk score to identify unrecognized patient subgroups, who have been previously misclassified by current risk stratifiers.
Method: The Multiple Myeloma Research Foundation CoMMpass study genomics dataset, combining mRNA Seq and clinical data from more than 700 MM patients, allowed us to evaluate the prognostic value of demographic and clinical parameters, cytogenetics, and gene expression levels of APOBEC genes as well as inflammation-modulating cytokines in MM patients. We calculated hazard ratios and Kaplan-Meier survival estimates for all extracted features. Combining clinical variables that were significantly associated with PFS and OS, we then applied machine learning approaches to identify the most accurate classification model to define a new risk score that is easy to compute and able to stratify NDMM patients more accurately than cytogenetics-based classifiers. Based on a Kaplan-Meier survival curve analysis, we then evaluated the performance of our newly built EI score in sub-classifying of current multiple myeloma risk stratifiers.
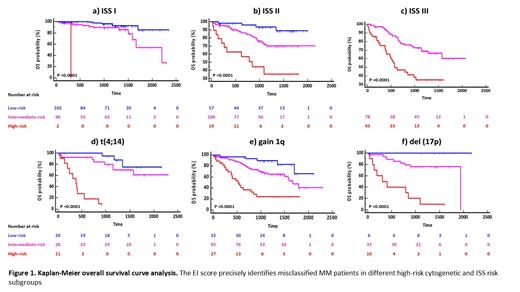
Results: Based on machine learning models, we defined a weighted OS/PFS risk score (Editor-Inflammation (EI) score) based on mRNA expression of APOBEC2, APOBEC3B, IL11, TGFB1, TGFB3, as well as ß2-microglobulin and LDH serum levels. We showed that the EI score subclassified patients into high-risk, intermediate-risk, and low-risk prognostic groups and demonstrated superior performance (C-index: 0.76) compared to ISS (C-index: 0.66) and R-ISS (C-index: 0.64). We further showed that EI low-risk patients do not benefit from autograft and maintenance therapy. Re-classification of ISS (Figure 1a, b, c) and R-ISS risk groups further confirmed the superiority of the EI score. In addition, the EI score identified previously unrecognized distinct subgroups of MM patients with adverse risk cytogenetics but good prognosis (Figure 1d, e, f). For example, the EI score excellently subclassified del(17p) MM patients into three main risk subgroups including a super low-risk group (none of them has p53 mut) with 5-year OS of 100%, an intermediate-risk group (30% of these patients also have p53 mut) with 5-year OS rate of 75%, and a very poor prognosis group of patients (40% of these patients also have p53 mut) with 5-year OS rate of 0% (2y OS: 40%) (Figure 1f). In line, we could show that patients with del(17p) and high EI score exhibit an enrichment of APOBEC induced genomic mutations compared to intermediate-risk and low-risk patients supporting the hypothesis that del(17p) along with high APOBEC expression levels activate the APOBEC mutation program and thus create an optimal environment for tumor progression. These findings support the necessity of a prognostic score that more accurately reflects MM disease biology.
Conclusion: Although MM is considered as an incurable disease, an improved risk stratification could help to identify previously unrecognized low- and high-risk patient subgroups that are over- or undertreated and lead to improved outcomes. Our EI score is a simple score that is based on recent insights into MM biology and accurately identifies high-risk and low-risk newly diagnosed MM patients as well as misclassified MM patients in different cytogenetic and ISS risk subgroups.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal